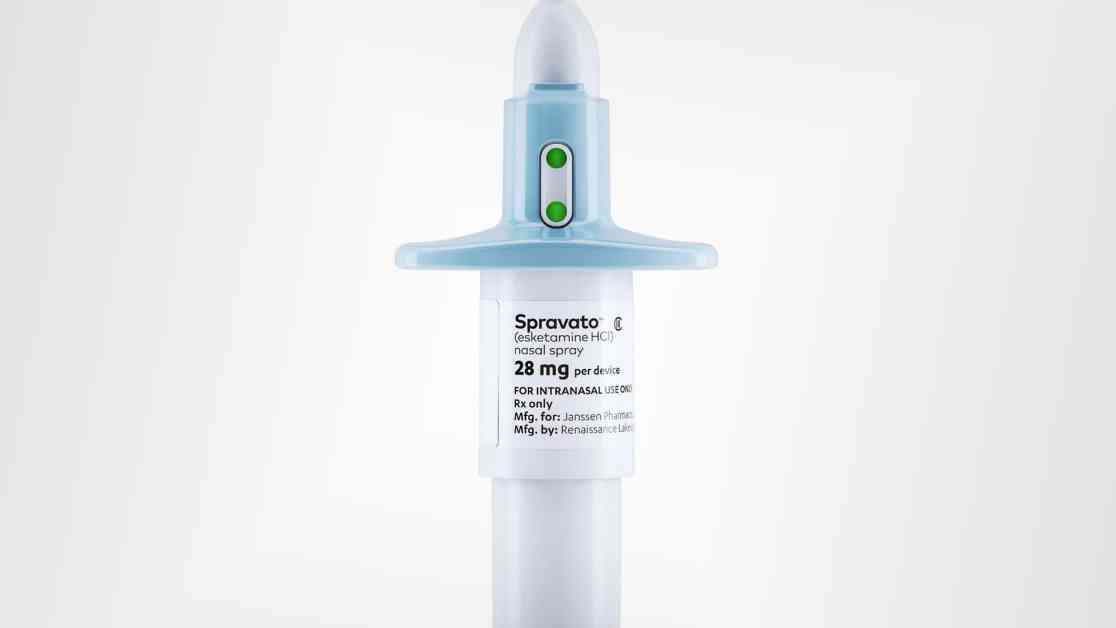
BREAKING NEWS: FDA Approves Johnson & Johnson Nasal Spray for Depression
The Food and Drug Administration has officially given the green light to Johnson & Johnson’s nasal spray, Spravato, for standalone use in adults grappling with treatment-resistant major depressive disorder. This groundbreaking decision marks a pivotal moment in the treatment of depression as Spravato becomes the first-ever standalone therapy for this challenging condition.
A Game-Changer for Millions
According to estimates, around one-third of the 21 million U.S. adults suffering from major depression find themselves in a relentless battle against symptoms like persistent sadness, sleep disturbances, low energy, and even thoughts of suicide. For these individuals, traditional treatments have fallen short, leaving them desperate for a solution that can provide the relief they so desperately seek.
Dr. Gregory Mattingly, a physician and president of the Midwest Research Group, hailed the approval of Spravato as a significant step forward in the fight against depression. Having been involved in the drug’s original clinical trials, he expressed optimism about the newfound freedom and hope that this treatment option offers patients who have struggled for so long.
A Beacon of Hope
Spravato’s journey to becoming a standalone treatment for depression has been a long and arduous one. Since its debut in 2019, the drug has gradually gained traction, with more doctors embracing its potential to transform the lives of those living with treatment-resistant depression. With $780 million in sales during the first nine months of 2024 and expectations of even greater growth in the future, Spravato is poised to become a game-changer in the mental health landscape.
J&J’s Bill Martin emphasized the importance of personalizing treatment paradigms for each individual, highlighting the significance of empowering patients to take charge of their health and well-being. By offering a new avenue for caregivers and patients to optimize their treatment plans, Spravato opens doors to a broader range of individuals who could benefit from its therapeutic effects.
The Road Ahead
As Spravato continues to pave the way for innovative approaches to depression treatment, the mental health community stands at the cusp of a new era in patient care. With real-world data supporting its efficacy and head-to-head studies showcasing its superiority over traditional antidepressants, Spravato is set to revolutionize how we address the complex challenges of major depressive disorder.
In the words of Dr. Mattingly, “We’ve all seen the benefits to our patients. So many of us have become really strong advocates for it.” With Spravato’s potential to offer rapid relief where other treatments have fallen short, the future looks brighter for individuals living with the burden of treatment-resistant depression.