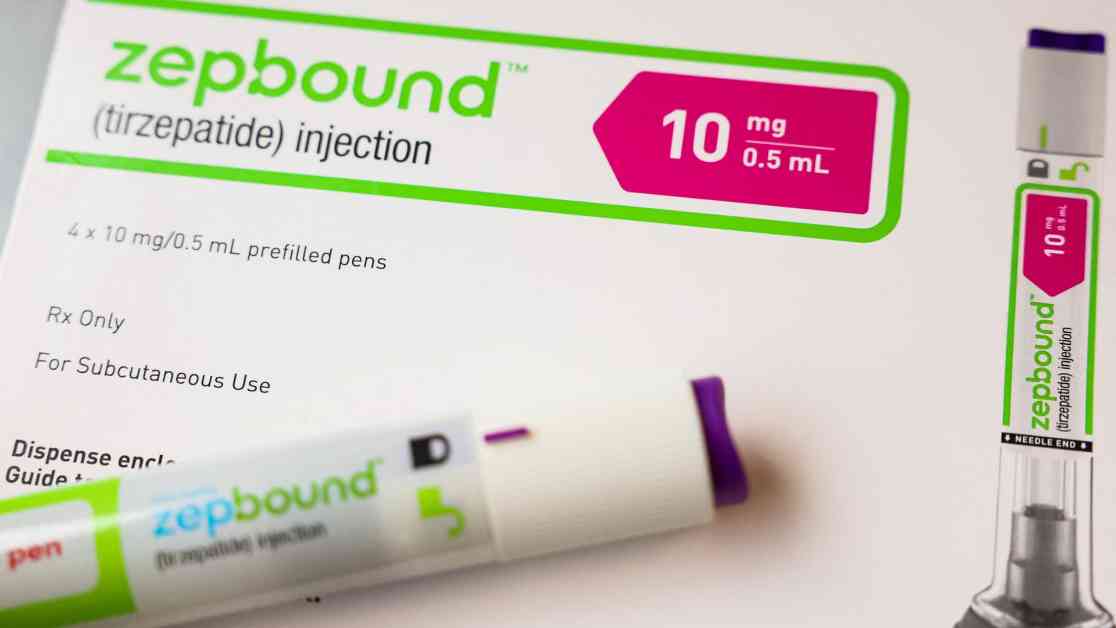
FDA Confirms Eli Lilly’s Weight Loss Drug Zepbound Availability
The Food and Drug Administration has officially confirmed the availability of Eli Lilly’s weight loss drug, Zepbound, marking a significant development in the ongoing dispute between the FDA and compounding pharmacies. Zepbound, which contains the active ingredient tirzepatide, has been the subject of intense scrutiny due to shortages that have plagued the market since December 2022.
Compounding Pharmacies to Cease Production of Unbranded Tirzepatide
Following the FDA’s announcement, compounding pharmacies will no longer be allowed to produce unbranded versions of tirzepatide, the active ingredient in Zepbound, within the next 60 to 90 days. This decision comes as a blow to pharmacies that have been providing affordable alternatives to patients who cannot access or afford the branded drug due to insurance coverage limitations.
High-Stakes Dispute and Legal Action
The decision to bar compounding pharmacies from producing unbranded tirzepatide follows a lawsuit filed by the Outsourcing Facilities Association against the FDA over the removal of tirzepatide from the official drug shortages list. The lawsuit alleged that the FDA’s actions favored Eli Lilly at the expense of patients and failed to address the ongoing shortage of tirzepatide.
Impact on Patients and the Market
Patients who rely on compounded versions of tirzepatide may face challenges transitioning to the branded drug due to cost and insurance coverage issues. The availability of affordable alternatives has been crucial for individuals struggling to access necessary medications amid rising prices and shortages in the market.
Despite the FDA’s efforts to regulate the production and distribution of tirzepatide, questions remain about the safety and efficacy of compounded medications compared to approved, branded alternatives. The agency has emphasized the importance of using FDA-approved medications when available to ensure patient safety and quality of care.
The decision to restrict compounding pharmacies from producing unbranded tirzepatide underscores the complexities of the pharmaceutical market and the challenges faced by patients, healthcare providers, and regulatory agencies in ensuring access to essential medications. As the industry continues to evolve, stakeholders must work together to address issues of affordability, availability, and patient safety to promote better health outcomes for all individuals.